.
Plants have been cultivated and studied from the earliest days
of human civilization, yet much remains unknown about them.
A good example is the mechanism by which the size of plant
cells is determined. Keiko Sugimoto, leader of the Cell Function
Research Unit in RIKEN’s Plant Science Center, in working to
elucidate this mechanism has discovered a series of genes that
control cell division or cell growth, attracting the attention of
researchers and companies worldwide. “Our research focuses
on the cellular aspects of plants,” says Sugimoto.
.
A garden of lilies
.
While a high-school student, Sugimoto noticed that a single lily that
had blossomed in her garden the year before had become three lilies
a year later, followed by ten the next year and as many as 100 the
year after. “But what impressed me most was that all of those flowers
were the same size, and had the same color and same shape every
year,” she says. “Although I knew that this was a manifestation of
heredity, which I had learned at school, I was fascinated. I wanted
to understand the mystery of plants, and this led me into research.”
.
.
.
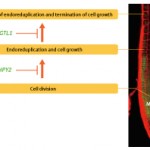
Figure 1. Mechanism underlying increases in plant size.
.
After completing her master’s course in Japan, Sugimoto gained
her PhD in plant science at the Australian National University. She
then went to work at the John Innes Center in the UK—a Mecca for
researchers studying plant biology—and in 2007 she set up the Cell
Function Research Unit in RIKEN’s Plant Science Center. “How is
plant cell size controlled? We are now working to solve this difficult
problem.”
.
The aspect that had impressed Sugimoto most as a high-school student
was that the sizes of flowers, leaves, seeds and other plant organs
depend roughly on the species of plant. Each organ grows as its cells
self-divide and increase in number, and each cell expands. However,
plant organs do not continue to grow infinitely. “Flowers and leaves
stop growing when they reach a certain size. Research has shown that
plant hormones such as auxin and cytokinin are involved in plant growth,
but we still don’t know how plant hormones control cell division and cell
expansion to determine ultimate organ size,” says Sugimoto. “Plant size
cannot be understood without knowing what is happening in cells. We
are conducting research focusing on the cellular aspects of plants,
which is a unique approach.”
.
Determinants of plant growth
.
“Every time I cut radishes or carrots into long, thin strips for cooking, I cannot
help admiring the slices for a moment,” says Sugimoto with a smile. “If you
look closely at a slice, you can see a finely textured portion near the tip. This
is called the meristem. It is dividing tissue where the cells self-divide. Try taking
a look next time you’re preparing a meal.”
.
In plants, meristems (Fig. 1) are found only in the tips of roots and stems. Plant
growth is the result of cell division and proliferation at the meristem, which is
followed by cell expansion. “There are two key time points in plant growth. One
is a turning point when cell division switches to cell expansion. Once a cell begins
expanding, it cannot return to the stage of proliferation by division. The other is the
point when cell growth stops and cells no longer expand. Plants cannot
grow normally unless these two points are strictly controlled.” Sugimoto and
her colleagues have attracted global attention for their discovery of the
genes that control these two growth points.
.
Genes control cell division and endoreduplication
.
Photograph showing a cross-section of Arabidopsis roots.
Plant cells divide mainly in the meristems at the tips of roots
and stems. After dividing several times, cells begin to increase
their size due to endoreduplication. When the cell reaches a
certain size, endoreduplication and cell growth cease. The
HPY2 gene controls the transition from cell division to
endoreduplication, whereas the GTL1 gene stops cell growth.
The green fluorescence indicates the HPY2 expression.
.
.
.
 .
.
.
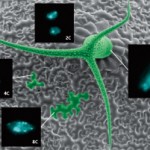
Figure 2. Endoreduplication and cell size in Arabidopsis.
.
.
A scanning electron microscopy image of the surface of an
Arabidopsis leaf showing a trichome. Most cells have 2C nuclear
DNA content, but some cells have increased nuclear DNA contents
of 4C, 8C and 32C due to endoreduplication. The four insets show
the correlation between cell size and the amount of nuclear DNA.
The trichome has a nuclear DNA content of 32C
.
.
Sugimoto used a new technique to discover genes that control the
Point at which cells stop dividing and begin increasing in size. “Many
researchers have tried to look for genes that control cell size, but most
of them were trying to find mutants with altered cell size. Mutants have
been identified merely based on the appearance of cells. We developed
a method to accurately measure nuclear DNA content and isolate
mutants with altered DNA levels.”
.
The cells of Arabidopsis, a commonly used experimental material in
plant science, just like human cells, have two sets of chromosomes,
one from the mother and the other from the father. The DNA of a cell
having two sets of chromosomes is denoted 2C. When a 2C cell
divides, its DNA is first replicated to produce 4C, which is then equally
distributed into the next two dividing cells, resulting in two 2C daughter
cells. In Arabidopsis, however, 2C and 4C cells are not the only cell
types to be found. Gametes (pollen, ovules) that have undergone
meiosis, a special process of cell division that results in half the
number of chromosomes as found in somatic cells, are 1C cells, but
there are also 8C, 16C and 32C cells (Fig. 2). “In plant cells, DNA
replication is sometimes followed by doubling in DNA without mitosis,”
says Sugimoto. “This phenomenon is called endoreduplication,
which results in 8C, 16C and 32C cells. The nuclear DNA and
cell size are correlated; cells expand as their nuclear DNA
increases.”
.
Together with Takashi Ishida, a postdoc in her lab, Sugimoto examined
the nuclear DNA content of Arabidopsis cells and discovered a mutant
having fewer 2C and 4C cells and more 32C, 64C and 128C cells (Fig. 3).
“Usually in plants, 2C and 4C cells in meristems continue to divide at a
constant rate. We assume that in the mutant we discovered, these
meristematic cells have undergone endoreduplication and switched into
cell expansion prematurely. A more detailed investigation revealed that
this mutant had lost the function of the HPY2 gene. Hence, HPY2 plays
a role in controlling the point of switching to endoreduplication, where
cells stop dividing and instead grow in size.”
.
 .
.
.
.
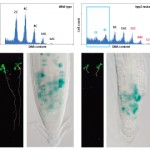
Figure 3. Regulation of cell division by the HPY2 gene.
.
This achievement was announced in August 2009, drawing attention
not only from plant biologists, but also from researchers studying a wide
variety of other organisms. “This is because HPY2 is involved in the
function of a small peptide known as SUMO, a small ubiquitin-like
modifier. SUMO is found in a broad range of species, from humans to
plants and yeasts. It binds to other proteins to enhance or weaken their
functions, and to regulate the diverse functions of cells. The reason
why my result attracted so much attention is that researchers studying
diverse ranges of organisms have been interested in SUMO.”
.
Sugimoto’s group demonstrated that the protein produced by HPY2
mediates the binding of SUMO to other proteins, resulting in the regulation
of cell division. This was the first report of SUMO being associated with
the regulation of cell division in multicellular organisms. “I never thought
that my studies on the mechanism of plant cell size control would lead
to SUMO. Research is fascinating because it can lead to unexpected
results.”
.
Sugimoto has also discovered three other genes that control the switch
into endoreduplication like HPY2. Her next task is to clarify the differences
in their functions.
.
A gene terminating cell growth
.
In September 2009, following the discovery of HPY2, Sugimoto’s group
discovered a gene involved in the second point—when plant cells stop
growing in size. “It began with the discovery of a mutant having very large
trichomes by Christian Breuer, a posdoc in my lab, who was searching
for mutants with abnormal cell size.”
.
Trichomes are hair-like outgrowths that cover the surfaces of Arabidopsis
leaves to protect them from insects, pathogens and even ultraviolet radiation
(Fig. 4). “Each trichome comprises a single epidermal cell in Arabidopsis.
It is large enough to be seen macroscopically.” While even a normal-sized
trichome is 500 times larger than an ordinary cell, the mutant discovered
by Breuer has trichomes that are more than twice this size.
.
.
 .
.
em>Figure 4: Arabidopsis trichomes.
(Upper) Nuclear DNA content of a cell population. The hpy2
mutant has lower ratios of 2C and 4C and higher ratios of 32C,
64C and 128C compared to the wild-type control. (Lower)
Photographs of wild-type (left) and hpy2-mutant (right) plants
ten days after germination. The mutant has very small roots
and leaves. The Blue staining indicates defective cell
division in the mutant.
.
The mutant was found to have the GTL1 gene partially modified and
expressed in excess. When the function of GTL1 was artificially
suppressed, the mutant’s trichomes became more than twice the size
of wild-type trichomes. Based on these experiments, Sugimoto’s group
hypothesized that GTL1 functions to terminate cell growth. To test the
hypothesis, they examined when and where GTL1 is expressed. It was
found not to be expressed in smaller trichomes in the early stage of
growth or trichomes that had stopped growing, but to be expressed
only in trichomes that have just expanded to maximum size (Fig. 5).
.
Previously, it had been thought that cell growth ceases when the supply
of cellulose and other components of the cell wall is stopped, or when
water absorption in vacuoles ceases. However, the discovery of GTL1
shows that plants have an intrinsic mechanism for actively stopping
cell growth. The discovery is groundbreaking, overturning the traditional
concept of plant growth.
.
Trichomes are cells undergoing endoreduplication, which is known to cease
at 32C. Sugimoto’s group is conducting research on the hypothesis that
GTL1 may control endoreduplication. It is already known that the function
of the gene necessary for endoreduplication is activated in mutants
lacking the function of GTL1. “GTL1 produces a protein known as a
transcription factor, which binds to the DNA of a certain gene to promote
or suppress its transcription to RNA. In the future, I want to clarify how
GTL1 controls transcription and of which genes, and to discover the
mechanism of endoreduplication.”
.
Giant prospects
.
Since the announcement of the discovery of GTL1, Sugimoto has received
a flood of offers for joint research, including many inquiries from industry,
who have great expectations for creating larger fruits and vegetables by
suppressing the function of GTL1.
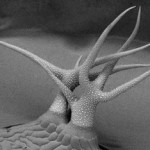
Trichomes are hair-like outgrowth at the plant leaf surface, each
comprising a single cell. These cells protect plants against insects
and pathogens, and trichomes in some species produce useful
secondary metabolites such as aspirin. Sugimoto’s group examined
Arabidopsis mutants with abnormally expanded trichomes and
discovered GTL1, a gene that terminates cell growth. The photograph
shows a mutant having an increased number of trichome branches.
A normal trichome has three branches.
.
.
 .
.
.
.
Figure 5. Trichome size and GTL1 expression..
.
.
Some cultivars are already available with increased yields thanks to artificial
duplication of nuclear DNA with chemical agents. However, this chemical
treatment unavoidably duplicates the nuclear DNA in all cells constituting the
plant body, which in turn makes the plant unable to produce seeds. “Advanced
research on GTL1 may allow us to promote endoreduplication at desirable
portions of plants, such as fruits, flowers and leaves, or whenever needed,
to change their sizes without preventing seed production,” says Sugimoto,
who is keen to conduct joint research with industry.
.
“Now is the most enjoyable time in my academic career,” declares Sugimoto.
However, she is not satisfied with just discovering the genes that control
plant growth. Further extensive investigation of the functions of individual
genes is needed. It is also necessary to identify the targets of HPY2 and
GTL1 to determine on which genes and proteins they act. She is also
interested in the relationship between HPY2 and GTL1, and their
association with plant hormones. “Much remains to be done, and I have
not found the answer to my question about lilies when I was a high school
student. In the plant kingdom, there are so many unanswered questions.
This is why I am fascinated by plant research.”
Photograph (left) and fluorescence image (right) of wild-type and GTL1-lacking
mutants. GTL1 (labeled with green fluorescent protein) is expressed only
in trichomes that have just grown to maximum sizes, and not in younger
or older trichomes.