.
Challenges in isolating and synthesizing protein-bound sugar molecules
called N-glycans, which help stabilize insulin levels and modulate
antibody-dependent immune responses among many other important
processes in the body, has limited the investigation of their function and
interaction with cultured cells and dissected tissues. Now, a team led by
Yasuyoshi Watanabe and Satoshi Nozaki from the RIKEN Center for
Molecular Imaging Science (CMIS), Kobe, has developed the first series
of fluorescent and radioactive probes to track these molecules in living
animals, which may eventually be used to track tumors.
.
.
 .
.
.
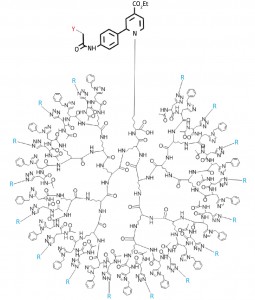
Figure 1: The structure of the tree-like cluster with 16 sugar branches
.
According to Nozaki, N-glycans, which contain sialic acid residues, always
form clusters in vivo allowing them to maximize their interactions and selectivity
towards N-glycan-binding proteins and other biomolecules. “It is rather rare that
a single molecule of N-glycan shows significant biological activity,” he says.
.
To recreate these in vivo conditions, the researchers worked in close collaboration with
Katsunori Tanaka from Osaka University to attach up to 16 sugar molecules to branched
lysine oligopeptides, creating the largest tree-like oligosaccharide cluster ever prepared
(Fig. 1). After linking the clusters to fluorescent and radioactive labels, they injected the
resulting probes into the tail vein of immunodeficient mice.
.
Positron emission tomography (PET) imaging showed that the number of glycans in the
clusters determined their lifetime in vivo. Four- and eight-sugar clusters rapidly disappeared
through the kidney in just one hour. Clusters containing 16 N-glycans, however, remained
in the body for over four hours before being eliminated through the kidney and the gallbladder
—a desirable feature when studying how N-glycans travel in living subjects.
.
Furthermore, the team discovered that differences in the way the sialic acids are connected to the
N-glycans influenced cluster behavior and build up in specific organs. The so-called (2–6)-linked
sialic acids stabilized the clusters in serum, leading to their accumulation in the liver through
interactions with specific protein receptors. In contrast, their (2–3)-linked congeners rapidly cleared
through the bladder. Also, fluorescence imaging revealed that clusters bearing both types of linkages
were most fluorescent in the spleen, suggesting their capture by a part of the immune system
called the reticuloendothelial system.
.
The researchers hope to use these clusters as molecular probes for tumors. They are also planning
to prepare clusters consisting of three to four different glycans in order to enhance the selectivity of
the probes toward tumors and specific organs. “Nobody has done it, but the data shows that we can
achieve it,” says Nozaki.

















